VP PLAN SCOPE.
The Validation Master Plan (VMP) is the most important documents because it describes the basis concept for your overall site validation programme. This interactive detailed document is a delight and simple to use. The generic template and attached SOP lead you through the whole process quickly and seamlessly. This thirty page document is suitable for all types of pharmaceutical /biotechnical / medical device / API, manufacturer and or processer. The VMP addresses processvalidation, facility validation, utility validation, equipment qualification and cleaning validation. The objective is to define responsibilities, outline your methods involved in the qualification and validation of your facility, define the areas and systems to be qualified and validated and to provide a programme for achieving and maintaining a validated status.
THE VP ROLE IN THE VALIDATION PROCESS.
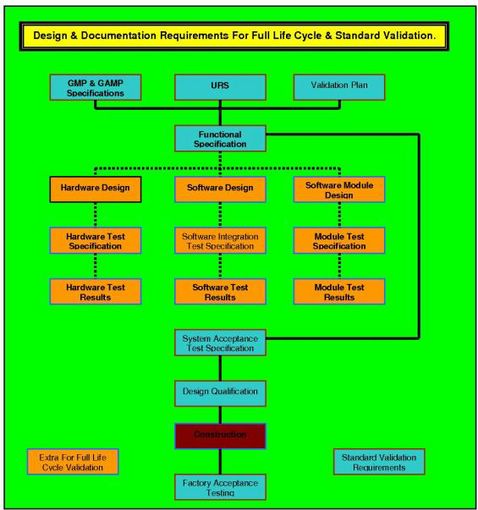
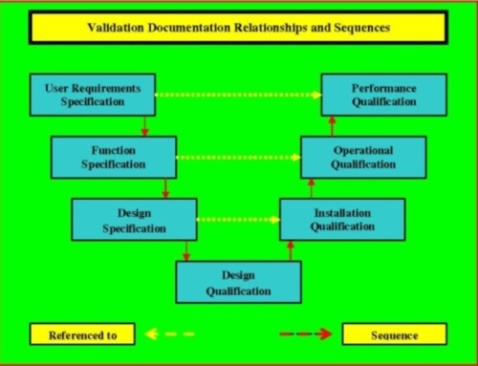
IT THEN PROGRESSES TO

No comments:
Post a Comment