P1Q is performed after successful completion of the Installation qualification (IQ)and Operational Qualifications (OQ) execution. The testing carried out is targeted at verifying that the performance specified in the URS is being delivered. Verification is also required to confirm the requirements specified in cGMP’s, health and safety rules and other guidance documents. Test objectives, acceptance criteria and methodologies must all be specified and pre-approved.
Performance Qualification (P1Q) is often used to qualify equipment throughout the full range of the equipment capabilities ,as opposed to process qualification, that is only concerned about capabilities that the process under validation uses.
P1Q SCOPE.
The normal expectations for Performance qualification (PQ) are given as requiring, documented verification that facilities, systems and equipment, as connected together, can perform effectively and reproducibly, based on the approved process method and product specification. Onto that now should be grafted The verification that the all the requirements specified in the User Requirements Specification (URS) have been fully complied with.
The P1Q represents the final qualification of your equipment or system. This incorporates a range of testing to simulate your production process options and provide assurance that your systems and your operating documentation, are capable of subsequent process validation activities. It is used to establish and or confirm;
The P1Q represents the final qualification of your equipment or system. This incorporates a range of testing to simulate your production process options and provide assurance that your systems and your operating documentation, are capable of subsequent process validation activities. It is used to establish and or confirm;
- Definition of performance criteria and test procedures.
- Selection of critical parameters, with predefined specifications.
- Determination of the test intervals, e.g.,
(a) - Everyday.
(b) - Every time the system is used.
(c) - Before, between and after a series of runs. - Define corrective actions on what to do if the system does not meet the established criteria.
P1Q RELATIONSHIPS.
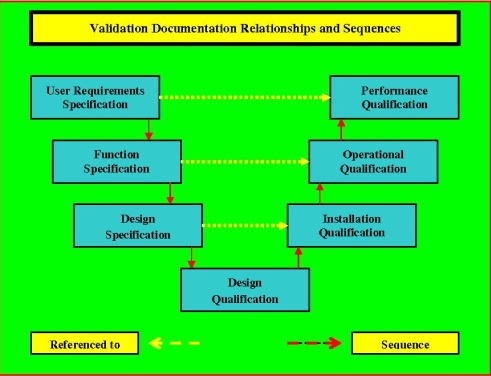

No comments:
Post a Comment