WHAT IS VALIDATION.
The guide-lines laid out in Good Automated Manufacturing Practices GAMP, GAMP 5, for the computer validation of automated systems including automatic computerized manufacturing equipment, control systems, automated laboratory systems, manufacturing execution systems and computers running laboratory or database systems. The automatic computerized system generally consists of the hardware, software and network components, together with the controlled functions and associated documentation. The recent release of GAMP 5 Procedures does not really over turn the previous release GAMP 4. Allthough GAMP 5 is definitely a handy guide, it does try to be all things to all people.
Which at times means certain sections can appear to contradict others.
On a recent project when a group was set up to develop a Risk Assessment (RA) document from GAMP 4, after four weeks they were still divided and unable to reconcile the requirements. It bodes well to always remember that GAMP 5 is a guide from the end users point of view. The actual legal requirements come from the regulators, and obviously takes precedence.
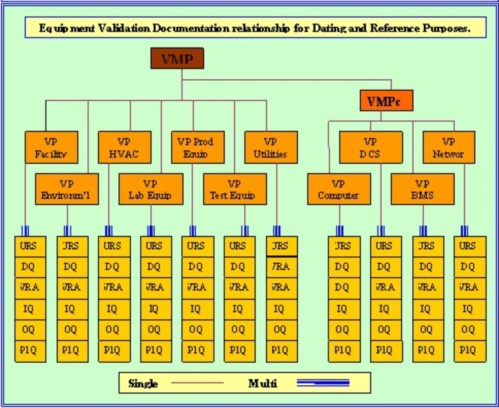
Validation Online's computer validation (CSV) protocols start with the development of a detailed three layer URS and progress through the VMP - IQ - OQ - PQ. Each of these documents are interactive and simple to use. Each are preceded with an SOP which guides the user through all phases of protocol generation.
The verification that the end users requirements as detailed in the URS have been fully satisfied, is paramount to the equipment being correctly qualified. Often this is hard to verify since the traceability from URS functionality to the actual hardware or software utilized is lost in the code generation stage.
However unless the effort is made to maintain this traceability, validation is flawed and future modifications can become problematic.
The Validation Master Plan - VMP, must be specific about this requirement and instruct all personnel involved in the qualification program, of the importance of maintaining this traceability.

No comments:
Post a Comment