VMP RATIONALE.
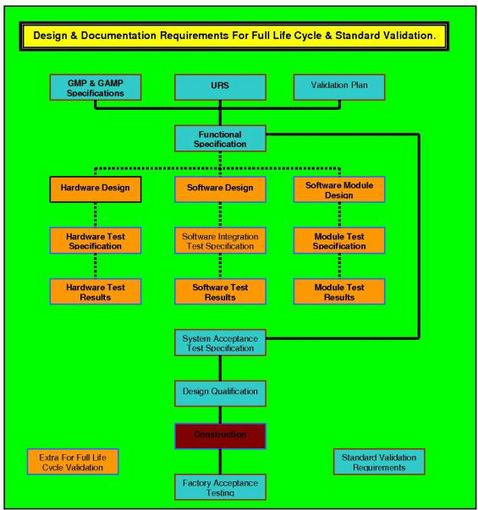
The VMP is a document that has never been mandatory, but is always one of the first documents a regulator asks to view. This is an extremely important document because in constructing it, many serious commitments and decisions have to be made. Program conceptions have to be mated to the User Requirements Specifications (URS), Level 1, 2 and or 3, these specifications have to be mated to the
Validation Master Plan (VP or VMP). From these plans the
Design Qualification (DQ),
Installation Qualification (IQ), the
Operational Qualification (OQ), and the
Performance Qualifications (PQ) have to be authorized, authored, approved for content, and issued for execution. The completed documentation has to be reviewed and accepted as complete by persons authorised to execute this role. All of these functions must be detailed in the VMP, when the project concept demands that avalidation master plan is required, or the Validation Plan (VP) where it does not. Responsibilities have to be declared, people have to be nominated, and everyone involved is duly served with a copy that carries the full authority of the company. The prospective progress of the project is there for everyone to compare to the actual progress. The VMP) is a document that documents the way the company will operate, who has control over the various aspects of the validation activities, and how production, quality control, and man management will be directed.
VMP SCOPE.
The document should cover the following subjects but should not limited to them alone:
- Introduction.
- Plan Origin and Approval.
- Derivation.
- Scope of Validation Activities.
- Validation Objectives.
- Validation Plan Review.
- Roles and Responsibilities.
- An Overview of Activities.
- Division of Responsibilities.
- System Description.
- Overview of System.
- Overview of Process.
- System Description.
- Validation Approach.
- Site Activities.
- Documentation and Procedures.
- Scope of Documentation.
- Validation Schedule of Activities.
- Project Master Schedule.
VALIDATION MASTER PLAN
IN THE QUALIFICATION PROCESS.


No comments:
Post a Comment