I have written before in these documents that certain things are critical, well here we are again. You have taken great trouble to write, and have approved, a URS and a VP (or could be VMP), now a vendor (or could be in house) has come forward and presented a design that they have prepared, and they state it will satisfy your requirements. This is where the majority of major project problems are manufactured, not obvious immediately, but materializing later in the project time line.
The Design Qualification is the only document that is going to confirm that the design will work. It must be carried out by qualified people who can challenge the design performance. If you have no such persons on your staff you must contract them in, or contract the DQ out.
When I arrive on site to manage a project, my very first task is always to get to grip with the design, get all the drawings and review them. I do this because thirty years of experience has made me very aware, that I need to know the design is good. So often this is not the case, and very often there are glaring abnormalities. When these are highlighted with the client and their vendors, only the vendors are smiling. The client had accepted the design and the vendor had quoted for that design, any changes will be extra to the quoted price. Sometimes this has run into seven figures.
The Design Qualification is the only document that is going to confirm that the design will work. It must be carried out by qualified people who can challenge the design performance. If you have no such persons on your staff you must contract them in, or contract the DQ out.
When I arrive on site to manage a project, my very first task is always to get to grip with the design, get all the drawings and review them. I do this because thirty years of experience has made me very aware, that I need to know the design is good. So often this is not the case, and very often there are glaring abnormalities. When these are highlighted with the client and their vendors, only the vendors are smiling. The client had accepted the design and the vendor had quoted for that design, any changes will be extra to the quoted price. Sometimes this has run into seven figures.
A PROPER DESIGN QUALIFICATION IS ESSENTIAL TO YOUR HEALTH.
A DQ can also be used where a company has prepared a User Requirements Specification (URS) for a piece of equipment and is searching for a manufacturer, but is offered equipment Of - The - Shelf. A DG can be used to verify whether the off-the-shelf item will fully deliver the functionality detailed in the URS, and conform to the requirements specified in the VMP / GAMP4 / cGMP and other Health and Safety Notices.
DQ SCOPE.
The scope of the DQ must include but is not limited to:- Verification that the design will achieve the URS requirements.
- Verification that the design is cGMP, and where software is used , conforms to the life cycle model requested in the VP and detailed in GAMP 4.
- Verification that the design complies with the VMP.
- Verification that the utility services required are available and validated.
- Verification that all the required support documentation is specified.
- Verification that the system will be calibratable.
- Verification that the system will be maintainable.
- Verification of operation staff training requirements.
- Verification that the system will operate in a manner safe to both product and staff.
- Verification that the system conforms to all applicable national standards and guidelines.
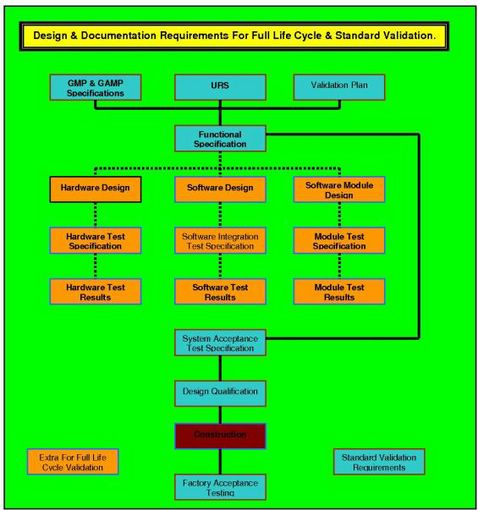
DQ IN THE VALIDATION PROCESS.

No comments:
Post a Comment