Validation Protocol standards must be used for writing the Functional Test (FT) if it is to be a stand alone document that can be executed and reviewed as part of the operational qualification. The FT should first be written at the FAT stage. This means that your vendor should write it, and so the vendor does in a high percentage of instances. It is criminal that having spent hundreds of hours authoring, developing and finally executing a detailed FAT at the factory, the document is allowed to die and never be used again. For instance in a project Validation Online was closely involved in, 87 people spent nearly one year in raising, approving and executing the FAT for a Distributive Control System, while on site, 30 people spent over a year writing the OQ’s. The FAT reviewed and condensed would have done a much better job, partly because it was written by staff who had direct access to the design staff who designed the DCS, but mainly because it was ready, and should have been freely available from the vendor. The FAT and the FT, are the same as the re-qualifying tests that are carried out on laboratory and process equipment. It becomes a little obvious that if these documents were written to normal validation protocol standards, then, the one document could be used for the testing section in documents like, the FAT, FT, qualify and re-qualifying of equipment.
Validation Protocol Standards
The following method of construction must be used. The over all protocol standards are shown in the SOP’ for the different protocols, here we are concerned about the testing element alone. All testing must be detailed and pre-approved by a qualified person to ensure the system under test has been adequately tested. Each test must comprise of ;
Main Sub-headings in Test Script.
- A Rationale, giving the reason and or object of the test.
- A detailed Test Method.
- A detailed Acceptance criteria that the tests must produce.
- A Test Result confirming whether the test result, satisfied the acceptance criteria.
General details that must be adhered to.- The test result must be initialled by the person executing the tests, on completion or at each significant stage.
- Each test must be designed to verify an element of the equipment functionality.
- Each test must a have a result that is clear, unambiguous and known.
- The test method must call up for the recording of the test result parameters. (no ticks or tick boxes, no generalities).
- Each test must be witnessed or the results must be reviewed by a competent person.
- The overall test results must be approve by a competent person.
STANDARD PROTOCOL RELATIONSHIPS.
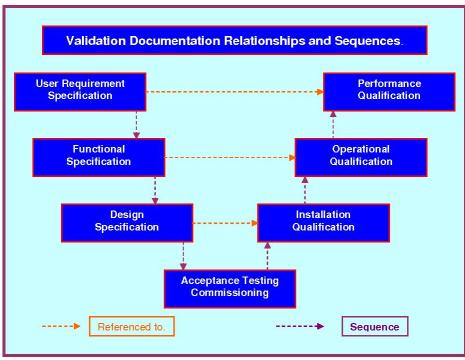
FULL LIFE CYCLE DOCUMENTATION RELATIONSHIPS.
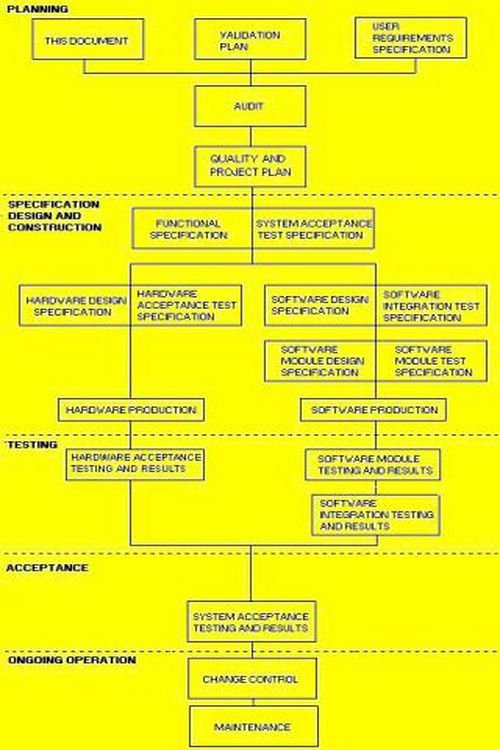



3 comments:
it help me to understand, documentation in pharma validation
The majority of this text is plagarized fro validation online.
PLEASE REMOVE BEFORE WE CONTACT GOOGLE TO GET YOU REMOVED.
Alex Kennedy
Validation Online since 2005.
The majority of this text is plagarized fro validation online.
PLEASE REMOVE BEFORE WE CONTACT GOOGLE TO GET YOU REMOVED.
Alex Kennedy
Validation Online since 2005.
Post a Comment