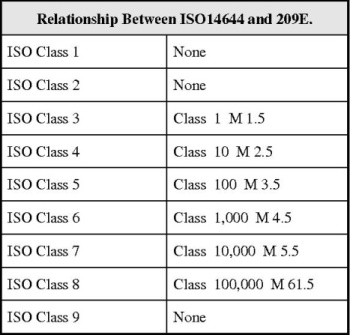
This HVAC Qualification, Design Qualification along with the attached Standard Operating Procedure, will, chapter by chapter take you through the task of raising a fully detailed DQ document. The main body is split into fourteen tables, each one probing the design requirements and standards for the individual requirement. Safety and security along with user operability are very detailed. The document will lead you through all these design aspects allowing you to delete some you feel are not important to your equipment. It is a superbly easy document to use and will ensure that you’re DQ’s are relevant, up to date and easy to execute. Practically all the requirements are in table form. Allowing fast and clearly presented results to be obtained.
HVAC QQUALIFICATION INSTALLATION.
This combined HVAC SOP and IQ template makes it so easy for you to raise a quality IQ. With the new suite of HVAC documents from the DQ - IQ - OQ - PQ, all user friendly and ready to go, your validation life has got much simpler. The final product is a professional and comprehensive Heating Ventilation and Air Conditioning Installation Qualification Protocol. One that you can produce in less than 60 minutes. Yes, think about it, we all know how long producing IQ documents has taken in the past. There is now no reason for not being able to produce 4 to 8, IQ protocols per 8 hour day, or a complete suite in one day.
GO TO STORE AND DOWNLOAD THESE DOCUMENTS NOW.
HVAC OPERATIONAL QUALIFICATION.
This easy to use HVAC Operational Qualification SOP and Protocol, can be simply and quickly converted (using find replace techniques) into your own company bespoke document. Now you can purchase these document in a suite DQ to PQ, getting you ready at express speed to qualify a system. It has never been quicker or simpler.

1 comment:
How many probe used for autoclave validation . if any guideline used for probe selection.
Post a Comment